Natural-looking
Dysport provides temporary improvement in the appearance of moderate to severe frown lines that are caused by the activity of the procerus and corrugator muscles in patients.1 Dysport helps them look consistently natural—not frozen—because it allows the untreated facial muscles to work normally, which in turn helps patients freely show facial expressions in untreated areas.

Individual results may vary.
Fast-acting
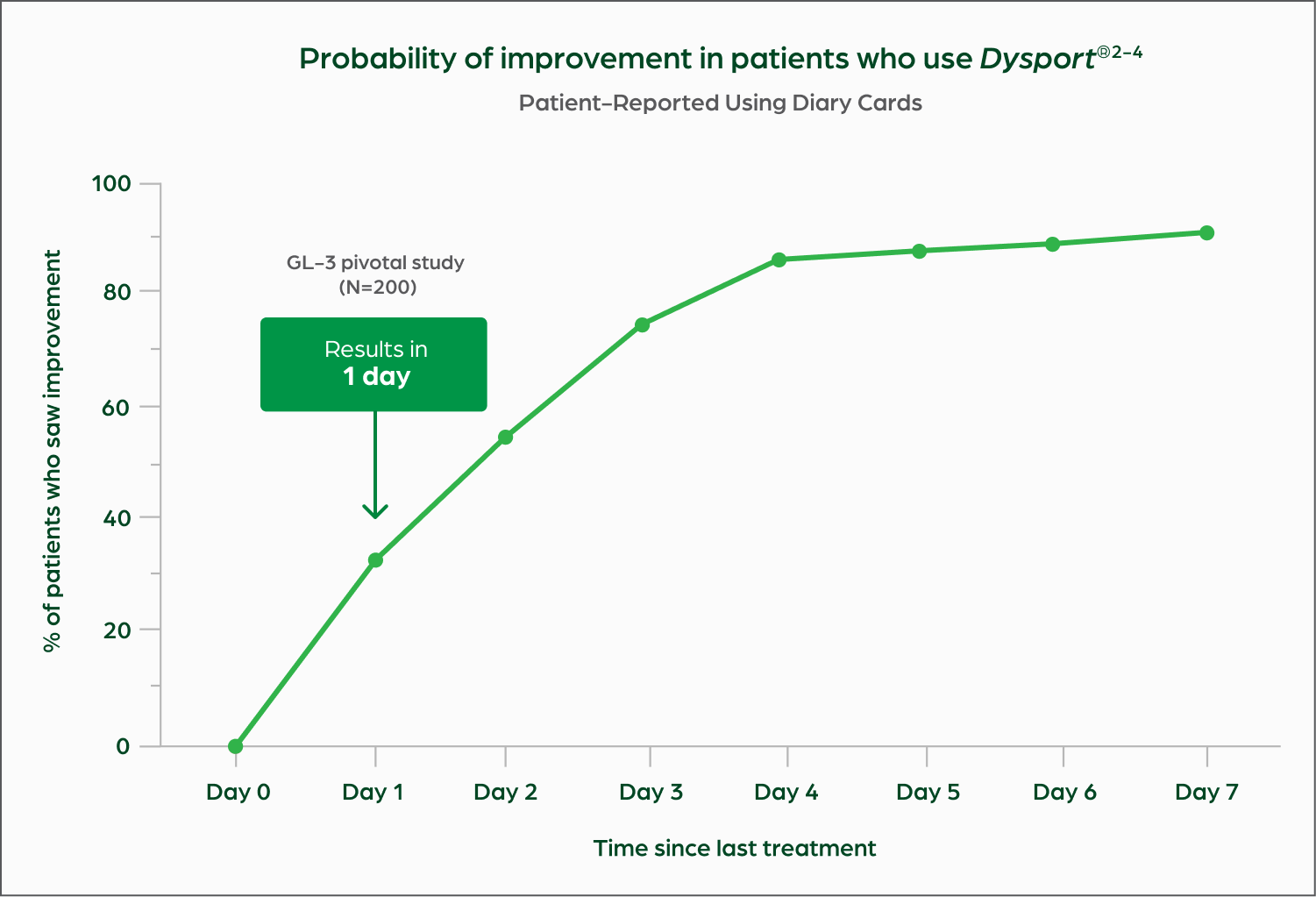
Dysport acts quickly for patients. Some users saw results within 24 hours, while more than half of users saw improvement in as little as 2-3 days after treatment with 50 units of Dysport versus placebo.2-5*†
* Users=clinical trial subjects.
† Based on subject self-assessment. The onset of response at day 1 was 15% (16/105) in GL-1 and 33% (65/200) in GL-3. In the DREAM study, the onset of response at day 1 was 33% (66/200). The median time to onset of response was 3 days in GL-1 (55/105; 52%) and GL-2 (36/71; 51%), and 2 days in GL-3 (110/200; 55%).
The GL-1 study was a 6-month, single-dose, double-blind, multicenter, randomized, placebo-controlled study (N=158) to assess the safety and efficacy of 50 units of Dysport vs placebo in subjects with moderate to severe glabellar lines at maximum frown.2 55% (58/105) of Dysport subjects vs 0/53 patients treated with placebo met the primary endpoint.
Long-lasting
Dysport delivered proven efficacy at every time point for up to 5 months.3,4,6* Moreover, the experience proved beneficial for users, with 98% of users† wanting to receive another Dysport treatment following 2 treatments.5
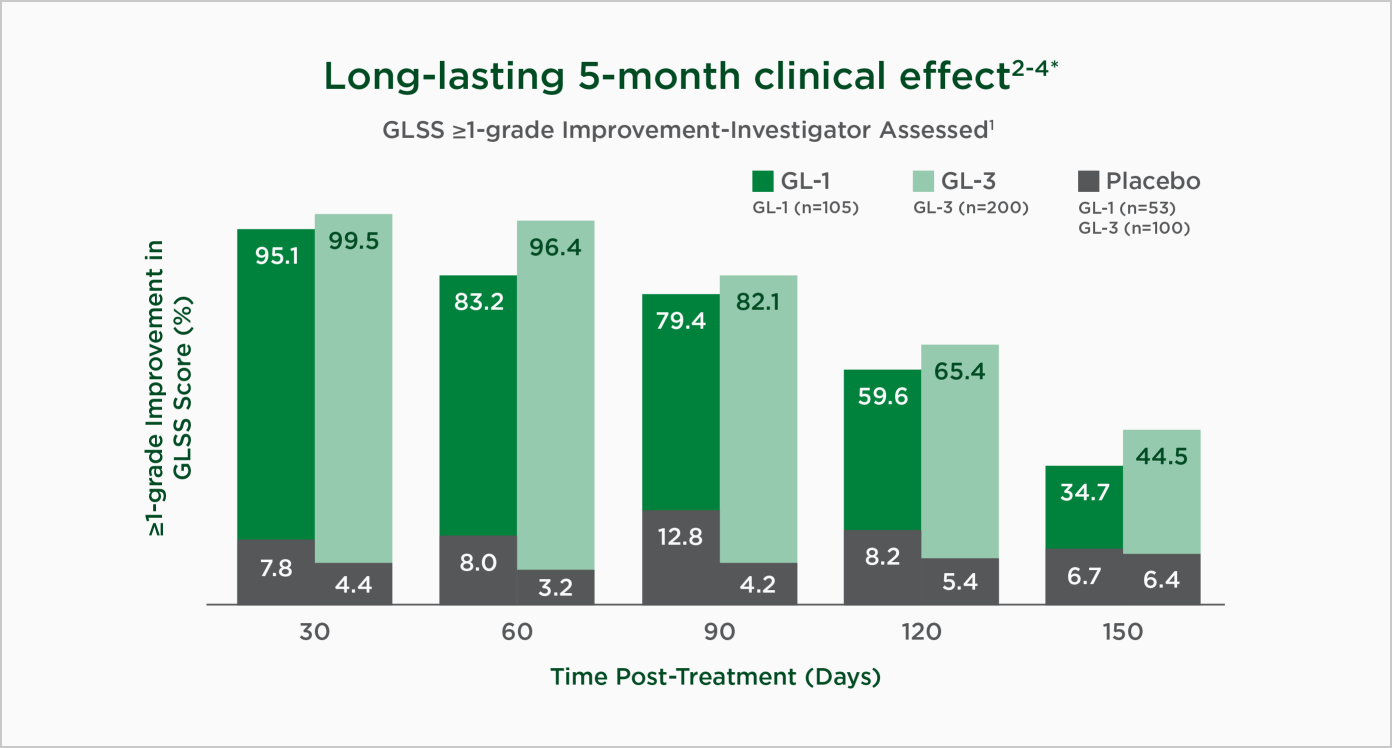
* GL-1 and GL-3 evaluated subjects once a month for at least 5 months following treatment. Based on a ≥1-grade GLSS improvement from baseline utilizing data from two double-blinded, randomized, placebo-controlled pivotal studies (GL-1, GL-3) in a post-hoc analysis. On average, efficacy was 97% at month 1, 90% at month 2, 81% at month 3, 63% at month 4, and 40% at month 5.
† Users=clinical trial subjects.
A well-established safety profile
Dysport has long-term safety results studied over three years5
- Well tolerated
- Low ptosis rate
- No cumulative effect of repeated treatments2,3,9
- Decreased incidence of treatment/injection-related reactions over repeated cycles

Definitely Dysport means real results
-
98%of users wanted to receive treatment again after 2 treatments.5*†
-
97%of toxin-naive users were satisfied after receiving 2 treatments, 6 months apart.6*§
-
97%of users felt their results looked natural.5*†
-
95%of users felt confident, attractive, and happy after treatment.5*†‡
*Users=clinical trial subjects.
†Subject-reported at 12 months (N=120) after two treatments
6 months apart in a phase IV, multicenter, prospective study.
‡3 out of 10 FACE-Q questions about psychological function are shown.
§Subject-reported at 12 months (N=34/35) after two treatments 6 months apart in a phase IV, multicenter, prospective study.
Build your business with ASPIRE Galderma Practice Rewards
Empower business growth. Strengthen patient relationships.
ASPIRE Galderma Practice Rewards is a program designed to strengthen the relationship you build with your aesthetics patients. As your connections grow, so does your practice,
and we’re here to support both with valuable insights, product offers, and practice tools.
GAIN Training
Easy to administer and reconstitute
Learn how to dose Dysport for moderate to severe glabellar lines.
We invite healthcare professionals to download this Dysport Reconstitution Guide or watch the video below to learn the proper application and administration methods, dosage amounts, and reconstitution process for Dysport treatment.
Connect with Us
Contact Galderma:
Call: 1-800-221-1749
Galderma Sales team representatives are available Monday to Friday,
from 8:00AM to 5:00PM CT
If you're a Healthcare Provider interested in learning more or using our
products, please provide your contact information at the link below.
Find a Specialist
The “Find a specialist” feature on DysportUSA.com makes it easy for patients to contact you regarding aesthetic treatment. To remain on the Dysport "Find a specialist" feature, please place an order for Dysport every 6 months at minimum.
- Dysport Prescribing Information. Galderma Laboratories, L.P. Dallas, TX. 2023.
- Rubin MG, et al. The efficacy and safety of a new U.S. Botulinum toxin type A in the retreatment of glabellar lines following open-label treatment. J Drugs Dermatol. 2009;8(5):439-444.
- Monheit GD, et al. Efficacy, Safety, and Subject Satisfaction After AbobotulinumtoxinA Treatment for Moderate to Severe Glabellar Lines. Dermatol Surg. 2020;46(1):61-69.
- Brandt F, et al. Randomized, placebo-controlled study of a new botulinum toxin type a for treatment of glabellar lines: efficacy and safety. Dermatol Surg. 2009;35(12):1893-1901.
- Schlessinger J, et al. A Multicenter Study to Evaluate Subject Satisfaction With Two Treatments of AbobotulinumtoxinA a Year in the Glabellar Lines. Dermatol Surg. 2021 Apr 1;47(4):504-509.
- Data on file. MA-35497. Post Hoc Analysis. Fort Worth, TX: Galderma Laboratories, L.P., 2017.
- Data on file. Ipsen World-Wide Marketing Authorisation Status. Fort Worth, TX: Galderma Laboratories, L.P., February 2017.
- Medicines and Healthcare products Regulatory Agency (MHRA). Botulinum Toxin Type A Powder For Solution For Injection (Clostridium botulinum toxin type A – Haemagglutinin complex). https://www.medicines.org.uk/emc/product/7261/smpc. Accessed June 4, 2025.
- Cohen JL, et al. An analysis of the long-term safety data of repeat administrations of botulinum neurotoxin type A-ABO for the treatment of glabellar lines. Aesthet Surg J. 2009;29(6 Suppl):S43-49.
Please see full Important Safety Information
for Dysport, for frown lines between the brows,including Distant Spread of Toxin Effect Boxed Warning at bottom of page.Please see full Important Safety Information
for Dysport, for frown lines between the brows, including Distant Spread of Toxin Effect Boxed Warning at bottom of page.
Important Safety Information
Dysport® (abobotulinumtoxinA) is a prescription injection for temporary improvement in the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults less than 65 years of age.
Important Safety Information
What is the most important information you should know about Dysport? Spread of Toxin Effects: In some cases, the effects of Dysport and all botulinum toxin products may affect areas of the body away from the injection site. Symptoms can happen hours to weeks after injection and may include swallowing and breathing problems, loss of strength and muscle weakness all over the body, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, or loss of bladder control. Swallowing and breathing problems can be life threatening and there have been reports of death. You are at the highest risk if these problems are pre-existing before injection.
These effects could make it unsafe for you to drive a car, operate machinery, or do other dangerous activities.
Do not have Dysport treatment if you: are allergic to Dysport or any of its ingredients (see the end of the Medication Guide for a list of ingredients), are allergic to cow's milk protein, had an allergic reaction to any other botulinum toxin product, such as Myobloc®, Botox®, or Xeomin®, have a skin infection at the planned injection site, under 18 years of age, or are pregnant or breastfeeding.
The dose of Dysport is not the same as the dose of any other botulinum toxin product and cannot be compared to the dose of any other product you may have used.
Tell your doctor about any swallowing or breathing difficulties and all your muscle or nerve conditions such as amyotrophic lateral sclerosis [ALS or Lou Gehrig's disease], myasthenia gravis, or Lambert-Eaton syndrome, which may increase the risk of serious side effects including difficulty swallowing and difficulty breathing. Serious allergic reactions have occurred with the use of Dysport. Dry eye has also been reported.
Tell your doctor about all of your medical conditions, including if you have surgical changes to your face, very weak muscles in the treatment area, any abnormal facial change, injection site inflammation, droopy eyelids or sagging eyelid folds, deep facial scars, thick oily skin, wrinkles that can't be smoothed by spreading them apart, or if you are pregnant or breastfeeding or planning to become pregnant or breastfeed.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins and herbal and other natural products. Using Dysport with certain other medicines may cause serious side effects. Do not start any new medicines while taking Dysport without talking to your doctor first.
Especially tell your doctor if you: have received any other botulinum toxin product, such as Myobloc® (rimabotulinumtoxinB), Botox® (onabotulinumtoxinA), or Xeomin® (incobotulinumtoxinA), in the last four months or any in the past (be sure your doctor knows exactly which product you received), have recently received an antibiotic by injection, take muscle relaxants, take an allergy or cold medicine, or take a sleep medicine.
Common Side Effects
The most common side effects are nose and throat irritation, headache, injection site pain, injection site skin reaction, upper respiratory tract infection, eyelid swelling, eyelid drooping, sinus inflammation, and nausea.
Ask your doctor if Dysport is right for you.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Dysport Full Prescribing Information including Medication Guide.




